Liver cancer is increasing globally, with hepatocellular carcinoma (HCC) as the most common primary liver cancer. Prognosis is highly stage-dependent: when HCC is detected early (≥2 cm, confined to the liver), five-year survival can exceed 70 per cent with curative therapies1. However, once vascular invasion occurs, survival drops below 15 per cent.
Early detection is therefore important, and gadoxetic acid-enhanced MRI (e.g., Eovist/Primovist) has emerged as the most sensitive modality for small HCCs, with an average sensitivity of 89 per cent, outperforming multiphasic computed tomography (CT) by about 10 per cent, and even more for tumours under 2 cm1,2. These diagnostic advantages have prompted wider use of MRI in at-risk populations, especially those with cirrhosis.
MRI protocols now exceed ultrasound in sensitivity for early-stage HCC screening and surveillance3. Given that earlier detection directly translates into better outcomes and access to treatment, MRI-based surveillance can help clinicians intervene sooner and extend survival for patients with liver disease.
Learn more about MRI for cancer detection here.
There are several reasons why a liver MRI might be ordered, including:
Unexplained Laboratory Abnormalities
Persistent elevation of liver enzymes (ALT or AST) or a rising tumour marker such as alpha-fetoprotein (AFP) or carcinoembryonic antigen (CEA) can signal underlying liver pathology, especially when neither ultrasound nor CT can provide a clear explanation4,5. MRI offers superior soft tissue characterisation, making it valuable in these ambiguous cases.
If prior imaging identifies a “mystery nodule”, particularly lesions 2 cm or smaller, nodules with arterial phase enhancement (“arterial blush”), or those labelled indeterminate (e.g., LI-RADS 3), MRI is the method of choice for detailed characterisation6. Advanced MRI sequences can distinguish between benign and malignant liver nodules7.
Individuals with cirrhosis (due to hepatitis B or C), hereditary hemochromatosis, Wilson disease, or carriers of cancer-predisposing mutations (such as TP53) are at elevated risk for developing liver cancer8. MRI is more sensitive than ultrasound for early detection and ongoing surveillance in these high-risk groups9.
When evaluating for metastatic spread, such as from colon, breast, pancreas, or melanoma, MRI can detect tiny liver metastases that might alter treatment plans10,11. Its contract resolution and ability to use liver-specific agents give it an advantage over CT or PET in many scenarios.
For patients undergoing interventions such as TACE (transarterial chemoembolization) or RFA (radiofrequency ablation), or those being evaluated for transplant or surgery, MRI helps accurately count and map tumours, assess for portal vein involvement, and gauge treatment response12,13.
Suspected complex vascular or biliary disorders, such as Budd-Chiari syndrome, arterio-venous shunts, cholangiocarcinoma, or intricate bile duct strictures, are best evaluated with MRI14. Its specialised sequences allow detailed visualisation of vessels and ducts, often clarifying diagnoses that ultrasound or CT cannot.
MRI plays a role in tracking metabolic or hormone-driven liver abnormalities, for example, monitoring hepatic adenoma growth in patients on long-term oestrogen or anabolic steroids, assessing cystic liver disease, or confirming a bile leak following abdominal trauma15,16.
Here are a few tips to help you prepare for your MRI17:
You can read more about preparation for Ezra’s Full Body Scan here.
Upon arrival for your MRI, you will need to check in and complete a screening form. This will allow you to confirm the presence of implants, allergies, and whether you might need any anxiety medication.
During the scan, you will lie down on a sliding table. A dedicated surface or phased-array coil is typically placed over the limb or region of interest19. The scan typically lasts 30-45 minutes of actual “table time”, during which the technician may acquire multiple sequences (settings), including localisers, axial and coronal T2 fast spin-echo images, in-phase and out-of-phase T1-weighted imaging to evaluate for fat and iron, diffusion-weighted imaging (DWI) with ADC maps, pre-contrast fat-suppressed 3D T1-weighted imaging, and dynamic post-contrast imaging using gadoxetic acid through arterial, portal-venous, and delayed phases, followed by a 20-minute hepatobiliary phase (HBP)20–23. Optional sequences may include MR elastography for liver stiffness, secretin-enhanced MRCP or standard MRCP for biliary anatomy, dynamic contrast-enhanced perfusion imaging to assess tumour response, and extracellular volume (ECV) quantification24–28.
You may be asked to hold your breath for short periods during the scan to minimise motion and improve image clarity.
You’ll hear a series of loud knocking or tapping sounds as the MRI machine works. This is completely normal. The scan usually takes about 20 to 45 minutes, and you’ll be offered earplugs or headphones to make the experience more comfortable.
You’ll stay in touch with the team via a two-way intercom and a squeeze bulb, allowing you to communicate or pause the scan if needed. If contrast is required, it’s injected halfway through, possibly causing a brief cool sensation. After the final sequence, the coil is removed, and you’re free to go.
At Ezra, our Full Body Plus scan takes around 60 minutes total, with 45 minutes of table time. Earplugs or headphones are available.
MRI is generally considered very safe when proper screening and protocols are followed, but certain risks and side effects should be understood:
A deeper dive into possible side effects (such as heat, headaches, and gadolinium deposition) is available in our full guide.
At Ezra, we employ a contrast-free approach using wide-bore T3 machines to deliver a comfortable scanning experience.
MRI reports for liver scans often include specialised terms. Here’s a guide to some common phrases and what they mean for your diagnosis:
Ezra provides a radiologist-reviewed report in a non-technical and easy-to-understand format on your dashboard.
After the MRI scan, you will be free to go home and continue with your day without any precautions39. If you received a sedative, you will need another person to pick you up. You will also not be able to drive, consume alcohol or operate heavy machinery 24 hours after the sedative.
A team of experts will review your results and determine whether a follow-up is necessary and recommend the appropriate treatment if needed. If abnormalities are found, you may undergo ongoing monitoring every 2-3 months to track recurrence. You can receive support in the form of counselling and advice on how to handle aspects like claustrophobia.
If you have a scan with us here at Ezra, you will receive your report within five to seven days and have the option to discuss it with a medical practitioner. You can also access your scan images through the online portal.
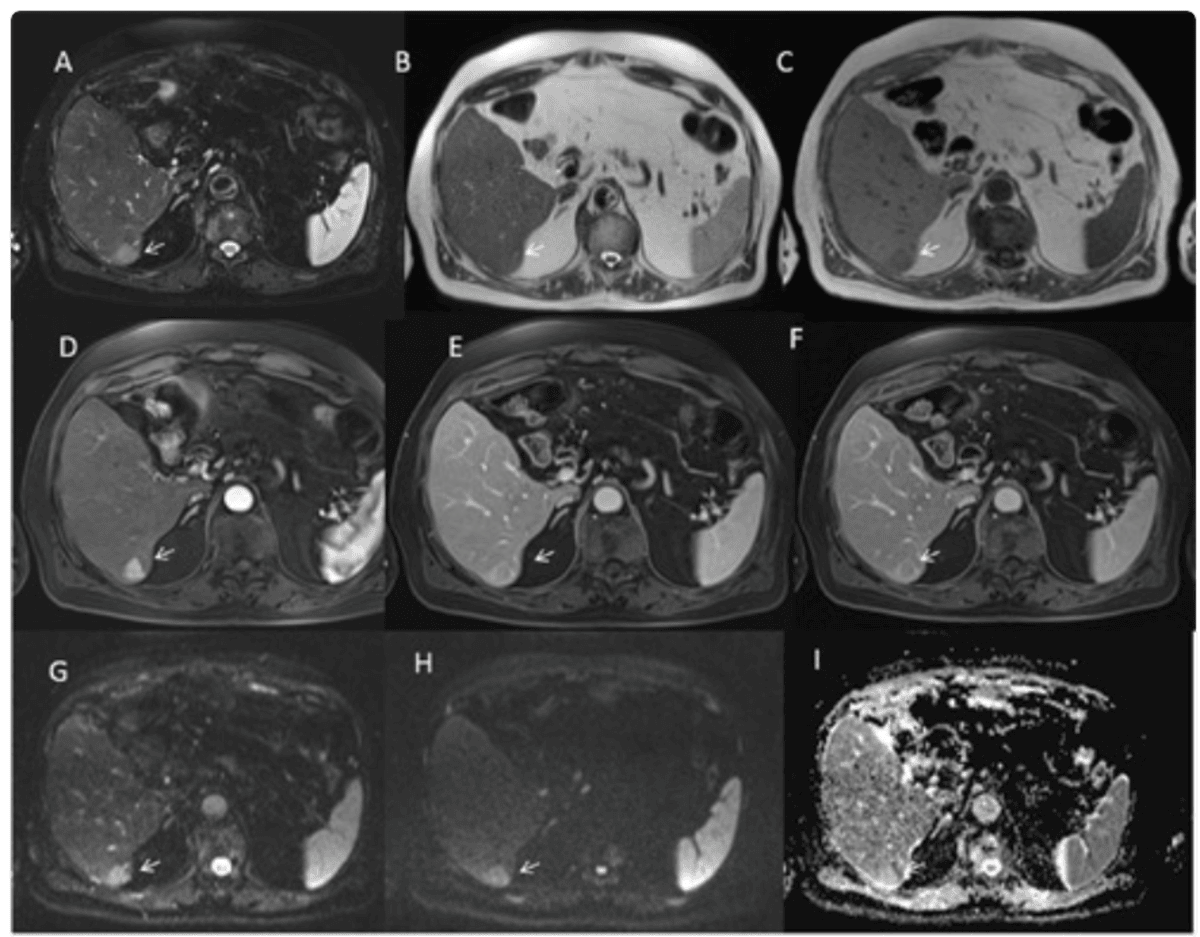
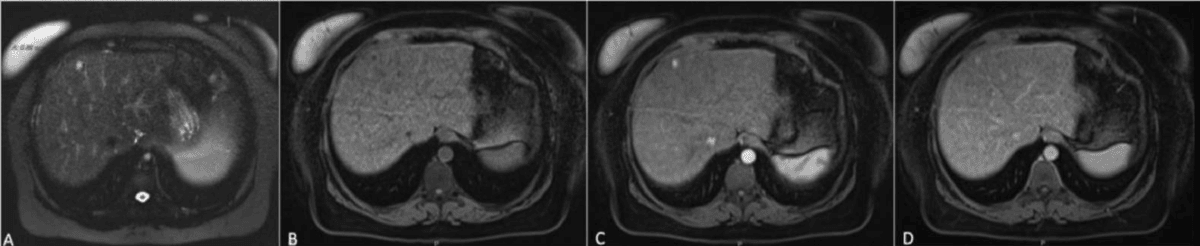
MRI can identify HCCs using a highly specific combination of features, known as the “triple sign”. These include arterial phase hyper-enhancement, wash-out, and capsule appearance40,41. The presence of all three findings strongly suggests HCC, especially in patients with cirrhosis or chronic liver disease.
When hepatobiliary contrast agents are used, healthy liver tissue takes up contrast and appears bright in the hepatobiliary phase, while malignant lesions, lacking functioning hepatocytes, remain dark. This finding is especially useful for identifying small (<1 cm) HCCs and distinguishing them from benign nodules or regenerative tissue. Lesions such as HCC or metastases are typically hypointense compared to the glowing background of the normal liver42–44.
Diffusion-weighted imaging (DWI) is highly sensitive to cellular structure. Malignant liver tumours, due to their high cellular density, restrict water motion, resulting in a markedly increased signal and corresponding low apparent diffusion coefficient (ADC) values. This property enables detection of HCCs and even very small metastases that might be missed by CT, and can help differentiate malignant from benign or treated lesions.
MRI is the reference standard for assessing vascular invasion, including detection of portal vein tumour thrombosis (PVTT)45. Identifying PVTT is critical since its presence may contraindicate liver transplantation or alter surgical planning. Features suggesting tumour thrombus include enhancement of the thrombus on post-contrast MRI and vessel expansion.
MR elastography non-invasively measures liver stiffness, a surrogate for fibrosis and risk of future decompensation46,47. Elevated liver stiffness on MR elastography is a strong predictor of adverse outcomes, including risk for developing new HCCs or liver failure after treatment. This measurement aids in prognostic stratification and monitoring treatment response for patients with chronic liver disease or cirrhosis.
Ezra utilises DWI as part of our whole-body MRI scans and artificial intelligence (AI) to enhance MRI images and convert radiology reports into layman's term translations.
Ezra screens for over 500 conditions, including liver abnormalities.
There are multiple types of MRI scans, all using different methods to give a better visualisation of liver tumours.
Ezra uses whole-body DWI imaging to get a full picture of the body and catch any potential abnormalities.
Ezra’s MRI Scan with Spine costs £2,395 and is currently available at their partner clinic in Marylebone, London and Sidcup, with more locations planned in the future. No referral is required, so you can book your scan directly without first consulting a GP or specialist. Most people pay out of pocket, as insurance typically does not cover self-referred scans, but you may be able to seek reimbursement depending on your policy.
Liver cancer often appears as a mass that is darker than normal liver on T1-weighted images, brighter on T2-weighted images and shows early contrast enhancement with “washout” in delayed phases.
A liver MRI with contrast typically takes between 10 and 30 minutes, with a total visit lasting up to an hour, including preparation time.
A liver MRI with contrast provides detailed images that help visualise liver structures, blood vessels, and abnormal growths, allowing for the assessment of both anatomical and functional changes. This makes it highly effective in detecting tumours and other liver diseases.
Typically, only your upper body is positioned in the MRI scanner for a liver MRI, not your entire body.
Liver cancer can be detected early through regular screening in high-risk individuals using ultrasound and/or blood tests for alpha-fetoprotein (AFP), as early liver cancer rarely causes symptoms.



1. Outcomes (prognosis) after HCC treatment. Liver Cancer UK. Accessed July 16, 2025. https://livercanceruk.org/liver-cancer-information/types-of-liver-cancer/hcc/treating-hcc/outcomes-prognosis-after-hcc-treatment/
2. Kim YY, Park MS, Aljoqiman KS, et al. Gadoxetic acid-enhanced magnetic resonance imaging: Hepatocellular carcinoma and mimickers. Clin Mol Hepatol. 2019;25(3):223-233. doi:10.3350/cmh.2018.0107
3. Chan MV, McDonald SJ, Ong YY, et al. HCC screening: assessment of an abbreviated non-contrast MRI protocol. Eur Radiol Exp. 2019;3:49. doi:10.1186/s41747-019-0126-1
4. EDOO MIA, Chutturghoon VK, WUSU-ANSAH GK, et al. Serum Biomarkers AFP, CEA and CA19-9 Combined Detection for Early Diagnosis of Hepatocellular Carcinoma. Iran J Public Health. 2019;48(2):314-322.
5. Gotlieb N, Schwartz N, Zelber-Sagi S, et al. Longitudinal decrease in platelet counts as a surrogate marker of liver fibrosis. World J Gastroenterol. 2020;26(38):5849-5862. doi:10.3748/wjg.v26.i38.5849
6. Shin SK, Kim YS, Choi SJ, et al. Characterization of small (≤3 cm) hepatic lesions with atypical enhancement feature and hypointensity in hepatobiliary phase of gadoxetic acid-enhanced MRI in cirrhosis: A STARD-compliant article. Medicine. 2017;96(29):e7278. doi:10.1097/MD.0000000000007278
7. Robinson PJA. Detecting and characterising small liver tumours. Cancer Imaging. 2003;3(2):85-87. doi:10.1102/1470-7330.2003.0005
8. Park SH, Kim B. Liver Magnetic Resonance Imaging for Hepatocellular Carcinoma Surveillance. JLC. 2020;20(1):25-31. doi:10.17998/jlc.20.1.25
9. Kim SY, An J, Lim YS, et al. MRI With Liver-Specific Contrast for Surveillance of Patients With Cirrhosis at High Risk of Hepatocellular Carcinoma. JAMA Oncol. 2017;3(4):456-463. doi:10.1001/jamaoncol.2016.3147
10. Sahani DV, Kalva SP, Fischman AJ, et al. Detection of Liver Metastases from Adenocarcinoma of the Colon and Pancreas: Comparison of Mangafodipir Trisodium–Enhanced Liver MRI and Whole-Body FDG PET. American Journal of Roentgenology. 2005;185(1):239-246. doi:10.2214/ajr.185.1.01850239
11. Maino C, Vernuccio F, Cannella R, et al. Liver metastases: The role of magnetic resonance imaging. World J Gastroenterol. 2023;29(36):5180-5197. doi:10.3748/wjg.v29.i36.5180
12. Lee JY, Lee BC, Kim HO, et al. Liver MRI and clinical findings to predict response after drug eluting bead transarterial chemoembolization in hepatocellular carcinoma. Sci Rep. 2021;11(1):24076. doi:10.1038/s41598-021-01839-6
13. Young S, Taylor AJ, Sanghvi T. Post Locoregional Therapy Treatment Imaging in Hepatocellular Carcinoma Patients: A Literature-based Review. Journal of Clinical and Translational Hepatology. 2018;6(2):189-197. doi:10.14218/JCTH.2017.00059
14. Brancatelli G, Vilgrain V, Federle MP, et al. Budd-Chiari Syndrome: Spectrum of Imaging Findings. American Journal of Roentgenology. 2007;188(2):W168-W176. doi:10.2214/AJR.05.0168
15. Río Bártulos C, Senk K, Schumacher M, et al. Assessment of Liver Function With MRI: Where Do We Stand? Front Med. 2022;9. doi:10.3389/fmed.2022.839919
16. Qi YM, Xiao EH. Advances in application of novel magnetic resonance imaging technologies in liver disease diagnosis. World Journal of Gastroenterology. 2023;29(28):4384-4396. doi:10.3748/wjg.v29.i28.4384
17. Radiology (ACR) RS of NA (RSNA) and AC of. Magnetic Resonance Imaging (MRI) - Head. Radiologyinfo.org. Accessed July 3, 2025. https://www.radiologyinfo.org/en/info/mri-brain
18. Williams JM, Hilmes MA, Archer B, et al. Repeatability and Reproducibility of Pancreas Volume Measurements Using MRI. Sci Rep. 2020;10:4767. doi:10.1038/s41598-020-61759-9
19. Gruber B, Froeling M, Leiner T, et al. RF coils: A practical guide for nonphysicists. J Magn Reson Imaging. 2018;48(3):590-604. doi:10.1002/jmri.26187
20. Donato H, França M, Candelária I, et al. Liver MRI: From basic protocol to advanced techniques. Eur J Radiol. 2017;93:30-39. doi:10.1016/j.ejrad.2017.05.028
21. Li J, Ma C, Chen Y, et al. The Feasibility of a Fast Liver MRI Protocol for Lesion Detection of Adults at 3.0-T. Front Oncol. 2021;11. doi:10.3389/fonc.2021.586343
22. Li H, Chen TW, Chen XL, et al. Magnetic resonance-based total liver volume and magnetic resonance-diffusion weighted imaging for staging liver fibrosis in mini-pigs. World J Gastroenterol. 2012;18(48):7225-7233. doi:10.3748/wjg.v18.i48.7225
23. Rosenkrantz AB, Patel JM, Babb JS, et al. Liver MRI at 3 T Using a Respiratory-Triggered Time-Efficient 3D T2-Weighted Technique: Impact on Artifacts and Image Quality. American Journal of Roentgenology. 2010;194(3):634-641. doi:10.2214/AJR.09.2994
24. Yin M, Ehman RL. MR Elastography: Practical Questions, From the AJR Special Series on Imaging of Fibrosis. American Journal of Roentgenology. 2024;222(1):e2329437. doi:10.2214/AJR.23.29437
25. Dillman JR, Trout AT, Taylor AE, et al. Association Between MR Elastography Liver Stiffness and Histologic Liver Fibrosis in Children and Young Adults With Autoimmune Liver Disease. American Journal of Roentgenology. 2024;223(1):e2431108. doi:10.2214/AJR.24.31108
26. Melekoglu Ellik Z, S. Idilman I, Kartal A, et al. Evaluation of Magnetic Resonance Elastography and Transient Elastography for Liver Fibrosis and Steatosis Assessments in the Liver Transplant Setting. Turk J Gastroenterol. 2022;33(2):153-160. doi:10.5152/tjg.2022.21705
27. Kim YC. Advanced Methods in Dynamic Contrast Enhanced Arterial Phase Imaging of the Liver. Investigative Magnetic Resonance Imaging. 2019;23(1):1-16. doi:10.13104/imri.2019.23.1.1
28. Schlaudraff E, Wagner HJ, Klose KJ, et al. Prospective evaluation of the diagnostic accuracy of secretin-enhanced magnetic resonance cholangiopancreaticography in suspected chronic pancreatitis. Magnetic resonance imaging. 2008;26(10):1367-1373. doi:10.1016/j.mri.2008.05.007
29. Gill A, Shellock FG. Assessment of MRI issues at 3-Tesla for metallic surgical implants: findings applied to 61 additional skin closure staples and vessel ligation clips. J Cardiovasc Magn Reson. 2012;14(1):3. doi:10.1186/1532-429X-14-3
30. Potential Hazards and Risks. UCSF Radiology. January 20, 2016. Accessed March 14, 2025. https://radiology.ucsf.edu/patient-care/patient-safety/mri/potential-hazards-risks
31. Costello JR, Kalb B, Martin DR. Incidence and Risk Factors for Gadolinium-Based Contrast Agent Immediate Reactions. Top Magn Reson Imaging. 2016;25(6):257-263. doi:10.1097/RMR.0000000000000109
32. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
33. Blum SFU, Ittermann T, Kromrey ML, et al. Long-term outcome of incidental cystic liver tumors in the general population. Sci Rep. 2021;11(1):11661. doi:10.1038/s41598-021-91140-3
34. Moreira-Silva H, Amorim J, Santos-Silva E. Incidental Liver Lesions in children: A practical and evidence-based approach. Clinics and Research in Hepatology and Gastroenterology. 2022;46(5):101904. doi:10.1016/j.clinre.2022.101904
35. Sawatzki M, Husarik DB, Semela D. Assessment of focal liver lesions in non-cirrhotic liver – expert opinion statement by the Swiss Association for the Study of the Liver and the Swiss Society of Gastroenterology. Swiss Medical Weekly. 2023;153(9):40099-40099. doi:10.57187/smw.2023.40099
36. Mall MA, Stahl M, Graeber SY, et al. Early detection and sensitive monitoring of CF lung disease: Prospects of improved and safer imaging. Pediatr Pulmonol. 2016;51(S44):S49-S60. doi:10.1002/ppul.23537
37. Fowler KJ, Bashir MR, Fetzer DT, et al. Universal Liver Imaging Lexicon: Imaging Atlas for Research and Clinical Practice. RadioGraphics. 2023;43(1):e220066. doi:10.1148/rg.220066
38. Radiology (ACR) RS of NA (RSNA) and AC of. How to Read Your Liver Imaging Report using LI-RADS. Radiologyinfo.org. Accessed July 16, 2025. https://www.radiologyinfo.org/en/info/article-lirads-liver-imaging
39. MRI scan. NHS inform. Accessed July 3, 2025. https://www.nhsinform.scot/tests-and-treatments/scans-and-x-rays/mri-scan/
40. Liu YI, Shin LK, Jeffrey RB, et al. Quantitatively Defining Washout in Hepatocellular Carcinoma. American Journal of Roentgenology. 2013;200(1):84-89. doi:10.2214/AJR.11.7171
41. Choi JY, Lee JM, Sirlin CB. CT and MR Imaging Diagnosis and Staging of Hepatocellular Carcinoma: Part II. Extracellular Agents, Hepatobiliary Agents, and Ancillary Imaging Features. Radiology. 2014;273(1):30-50. doi:10.1148/radiol.14132362
42. Harper KC, Ronot M, Wells ML, et al. Hypointense Findings on Hepatobiliary Phase MR Images. Radiographics. 2025;45(2):e240090. doi:10.1148/rg.240090
43. Vernuccio F, Cannella R, Meyer M, et al. LI-RADS: Diagnostic Performance of Hepatobiliary Phase Hypointensity and Major Imaging Features of LR-3 and LR-4 Lesions Measuring 10–19 mm With Arterial Phase Hyperenhancement. American Journal of Roentgenology. 2019;213(2):W57-W65. doi:10.2214/AJR.18.20979
44. Vernuccio F, Gagliano DS, Cannella R, et al. Spectrum of liver lesions hyperintense on hepatobiliary phase: an approach by clinical setting. Insights Imaging. 2021;12:8. doi:10.1186/s13244-020-00928-w
45. Gawande R, Jalaeian H, Niendorf E, et al. MRI in differentiating malignant versus benign portal vein thrombosis in patients with hepatocellular carcinoma: Value of post contrast imaging with subtraction. Eur J Radiol. 2019;118:88-95. doi:10.1016/j.ejrad.2019.07.008
46. Moura Cunha G, Fan B, Navin PJ, et al. Interpretation, Reporting, and Clinical Applications of Liver MR Elastography. Radiology. 2024;310(3):e231220. doi:10.1148/radiol.231220
47. Tada A, Nagai T, Kato Y, et al. Liver stiffness assessed by magnetic resonance elastography predicts clinical outcomes in patients with heart failure and without chronic liver disease. Eur Radiol. 2023;33(3):2062-2074. doi:10.1007/s00330-022-09209-0
48. Kim JH, Joo I, Lee JM. Atypical Appearance of Hepatocellular Carcinoma and Its Mimickers: How to Solve Challenging Cases Using Gadoxetic Acid-Enhanced Liver Magnetic Resonance Imaging. Korean J Radiol. 2019;20(7):1019-1041. doi:10.3348/kjr.2018.0636
49. Manfredi R, Barbaro B, Masselli G, et al. Magnetic resonance imaging of cholangiocarcinoma. Semin Liver Dis. 2004;24(2):155-164. doi:10.1055/s-2004-828892
50. Chu LC, Fishman EK. Pancreatic ductal adenocarcinoma staging: a narrative review of radiologic techniques and advances. Int J Surg. 2023;110(10):6052-6063. doi:10.1097/JS9.0000000000000899
51. Irizato M, Minamiguchi K, Fujita Y, et al. Distinctive imaging features of liver metastasis from gastric adenocarcinoma with enteroblastic differentiation: A case report. World J Radiol. 2025;17(2):104518. doi:10.4329/wjr.v17.i2.104518
52. Rogers T, Shah N, Mauro D, et al. Anastomosing hemangioma of the liver: An unusual variant in abdominal MRI imaging. Radiol Case Rep. 2022;17(12):4889-4892. doi:10.1016/j.radcr.2022.09.052
53. Khanna M, Ramanathan S, Fasih N, et al. Current updates on the molecular genetics and magnetic resonance imaging of focal nodular hyperplasia and hepatocellular adenoma. Insights Imaging. 2015;6(3):347-362. doi:10.1007/s13244-015-0399-8
54. Kanno H, Maruyama Y, Sato T, et al. Hepatocellular adenoma initially diagnosed as hepatocellular carcinoma with resistance to proton beam radiotherapy - A case report. Int J Surg Case Rep. 2021;83:105955. doi:10.1016/j.ijscr.2021.105955
55. Feng Z, Zhao H, Guan S, et al. Diagnostic performance of MRI using extracellular contrast agents versus gadoxetic acid for hepatocellular carcinoma: A systematic review and meta-analysis. Liver Int. 2021;41(5):1117-1128. doi:10.1111/liv.14850
56. Heo S, Choi WM. Non-Contrast Abbreviated Magnetic Resonance Imaging: A Cost-Effective Rookie in Hepatocellular Carcinoma Surveillance for Cirrhotic Patients. Gut and Liver. 2024;18(1):7-9. doi:10.5009/gnl230537
57. Kele PG, van der Jagt EJ. Diffusion weighted imaging in the liver. World J Gastroenterol. 2010;16(13):1567-1576. doi:10.3748/wjg.v16.i13.1567
58. Schambeck JPL, Forte GC, Gonçalves LM, et al. Diagnostic accuracy of magnetic resonance elastography and point-shear wave elastography for significant hepatic fibrosis screening: Systematic review and meta-analysis. PLOS ONE. 2023;18(2):e0271572. doi:10.1371/journal.pone.0271572