Magnetic Resonance Imaging (MRI) has become a cornerstone in cancer detection due to its ability to produce highly detailed images of soft tissue without exposing patients to ionising radiation. This makes MRI a safe and repeatable choice, especially for early detection and for populations needing frequent monitoring, such as those at high risk for certain cancers. The use of gadolinium-based contrast agents and multiparametric protocols, which combine T2-weighted, diffusion-weighted (DWI/ADC), and dynamic contrast-enhanced (DCE) sequences, has established MRI as the gold standard for identifying and characterising tumours in the prostate and breast1,2.
Recent advancements have introduced whole-body diffusion MRI, enabling the rapid and radiation-free detection of metastatic disease throughout the body, rivalling PET/CT in sensitivity for many cancer types3. MRI’s superior soft-tissue contrast and functional imaging capabilities make it particularly effective for diagnosing cancer at early, potentially curable stages. As a result, MRI plays a crucial role in both initial diagnosis and ongoing assessment, supporting precise treatment planning and improved patient outcomes.
Learn more about MRI for Cancer Detection
The human body primarily comprises water, around 55-75 per cent4. Water's chemical composition consists of hydrogen and oxygen atoms. Inside each hydrogen atom resides a small particle called a proton. Protons have a positive electrical charge and are sensitive to magnetic fields5,6.
MRI machines use large, powerful magnets to generate a strong magnetic field around the patient7. When a person is placed inside the machine, this field causes the hydrogen atoms in their body to align in a particular direction.
The MRI machine then sends radio frequency (RF) pulses, temporarily disrupting the alignment of these hydrogen atoms. Once the RF pulses stop, the hydrogen atoms realign, releasing energy as radio waves picked up by the MRI scanner.
Since tissues contain varying amounts of water (and therefore hydrogen atoms) and release energy at different rates, the MRI scanner can differentiate between tissue types and produce high-resolution cross-sectional images8.
An MRI scanner typically has a large, cylindrical tube-like structure. This is where the patient lies during the imaging process. Inside the MRI are7:
The MRI room typically has a control room where the technologist operates the machine. This area includes screens and buttons to control the MRI process and monitor the patient.
Computer and software systems control the MRI and radio wave pulses. These systems process the signals received from the coils and convert them into images.
MRI scans can be performed with or without a special dye called gadolinium:
Contrast MRI (with gadolinium):
Non-Contrast MRI (without gadolinium):
MRI in oncology leverages a range of sequences and techniques to improve the detection, characterisation, and staging of cancer. Each method provides unique tissue contrasts or functional information critical for accurate staging.
MRI is highly valued in oncology for its ability to detect and characterise a range of cancers across different organs with strong sensitivity, often outperforming other imaging techniques, especially in soft-tissue evaluation. Sensitivity varies by cancer type, MRI technique, and use of contrast agents, but advanced protocols achieve excellent diagnostic accuracy in many organs.
Magnetic Resonance Imaging (MRI) is a crucial modality for identifying benign tumours and distinguishing them from malignant lesions across organ systems. Its superior soft-tissue contrast, multiplanar capability, and versatility make it valuable in diagnosing and characterising non-cancerous masses.
MRI signs: Well-defined, smoothly marginated lesions; lack of significant surrounding swelling31,32.
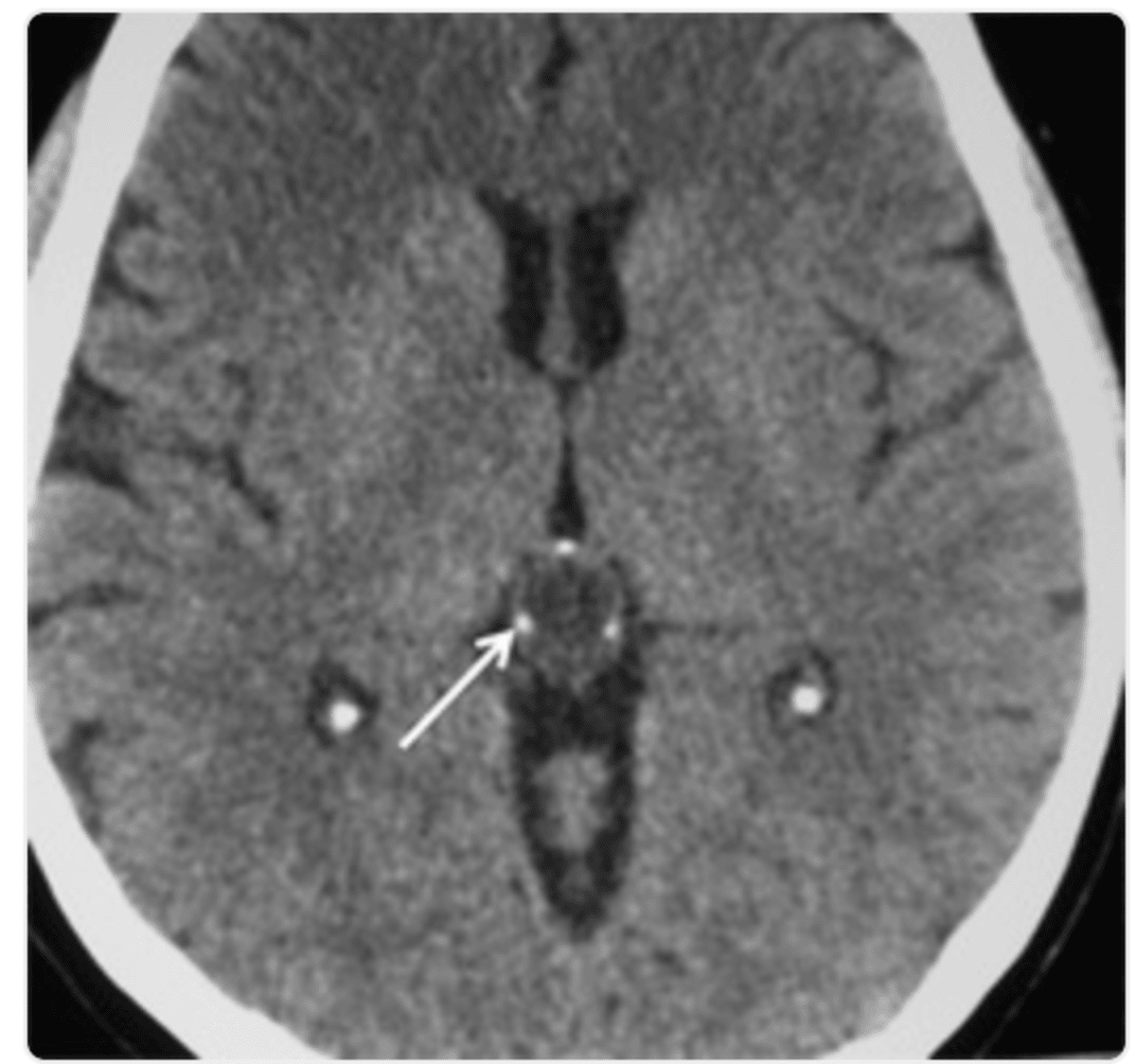
MRI signs: Well-circumscribed, even (homogenous) signal, often hyperintense on T233.
MRI signs: Homogenous fat signal (for lipomas), lack of invasion into surrounding structures34.
MRI signs: Characteristic signal properties (e.g., fat suppression for angiomyolipoma, vascular pooling for hemangioma), well-defined borders35,36.
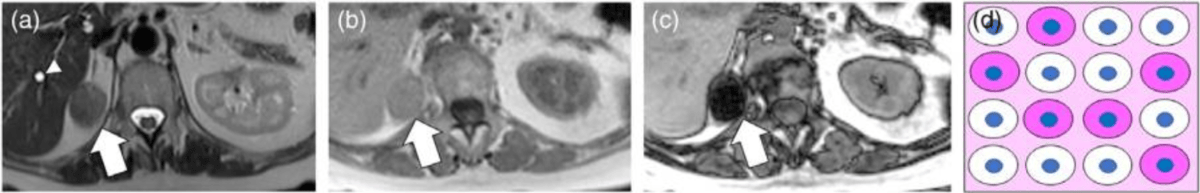
MRI signs: Well-defined margins, homogenous appearance, no cortical bone destruction, absence of soft tissue invasion37,38.
MRI is especially useful in children to avoid ionising radiation and to better characterise tissue properties and anatomical relations33,36,39.
Certain MRI features suggest a higher risk of malignancy (“red flags”) that should prompt histopathological confirmation via biopsy, even for lesions that might otherwise appear benign:
As shown above, MRI demonstrates high sensitivity and moderate to high specificity in cancer detection, which varies by tumor type and anatomical region. MRI is widely used for prostate cancer, where it efficiently helps distinguish clinically significant tumors44. In breast cancer, contrast-enhanced MRI serves as a reliable tool, often able to detect malignancies that may be subtle or inconspicuous using other modalities45.
In the evaluation of brain and head and neck tumors, MRI proves effective in revealing malignant lesions, with its accuracy influenced by both the specific imaging techniques employed and the site involved46. The diagnostic accuracy for differentiating tumour progression from treatment effects or benign conditions can be further enhanced by using a combination of multiple MRI techniques.
Certain lesions are prone to false negatives or underdiagnosis on MRI, such as:
This can hinder throughput and limit MRI availability for certain populations.
If you want to read more about the potential side effects from MRI and how these can be combated, read our article here.
Here are a few tips to help you prepare for your MRI53:
Upon arrival for your MRI, you will need to check in and complete a screening form. This will allow you to confirm the presence of implants, allergies, and whether you might need any anxiety medication.
During the scan, you will lie down on a sliding table. A dedicated surface or phased-array coil is typically placed over the limb or region of interest55. The scan typically lasts 30-45 minutes of actual “table time”, during which the technician may acquire multiple sequences (settings). You may be asked to hold your breath for short periods during the scan to minimise motion and improve image clarity.
You’ll hear a series of loud knocking or tapping sounds as the MRI machine works. This is completely normal. The scan usually takes about 20 to 45 minutes, and you’ll be offered earplugs or headphones to make the experience more comfortable.
You’ll stay in touch with the team via a two-way intercom and a squeeze bulb, allowing you to communicate or pause the scan if needed. If contrast is required, it’s injected halfway through, possibly causing a brief cool sensation. After the final sequence, the coil is removed, and you’re free to go.
After the MRI scan, you will be free to go home and continue with your day without any precautions56. If you received a sedative, you will need another person to pick you up. You will also not be able to drive, consume alcohol, or operate heavy machinery 24 hours after the sedative.
A team of experts will review your results and determine whether a follow-up is necessary, recommending the appropriate treatment if needed. If abnormalities are found, you may undergo ongoing monitoring every 2-3 months to track recurrence. You can receive support in the form of counselling and advice on how to handle aspects like claustrophobia.
If you have a scan with us here at Ezra, you will receive your report within five to seven days and have the option to discuss it with a medical practitioner. You can also access your scan images through the online portal.
Individuals with high-risk genetic profiles such as BRCA1/2 mutations or Lynch syndrome are recommended for regular MRI screening because of their significantly increased lifetime cancer risk. For example, annual breast MRI screening starting at age 25 is recommended for BRCA mutation carriers, as it enhances early cancer detection and improves survival rates57,58.
MRI colonography or colonoscopy is indicated for Lynch syndrome, and MRI is increasingly utilised for hepatocellular carcinoma (HCC) surveillance in populations at risk for liver disease, thanks to its superior sensitivity for detecting early-stage lesions compared to ultrasound59,60.
Symptom-driven MRI is warranted when clinical presentation or findings suggest conditions that require high-contrast, multiplanar imaging, such as unexplained neurological deficits, refractory headaches, spinal pain, or ambiguous mass lesions61. Correlating imaging findings with patient-reported symptoms substantially increases diagnostic accuracy, especially in cases with non-specific or overlapping clinical features.
MRI is preferred in children for complex or unclear diagnoses, but sedation is often necessary to ensure immobility, demanding specialised monitoring due to higher risks of respiratory and cardiovascular complications in the MRI setting62. During pregnancy, MRI is considered safe after the first trimester and is reserved for cases where a diagnosis will significantly impact management, with contrast usage minimised unless absolutely necessary63. In patients with renal failure, non-contrast MRI does not pose additional risk, but gadolinium-based contrast should be avoided or used with caution due to potential risk of nephrogenic systemic fibrosis.
Early detection of cancer with MRI leads to significantly better patient outcomes, as cancers identified at an early stage are more likely to be smaller, node-negative, and have higher survival rates compared to those found later. Studies demonstrate that high-risk cohorts undergoing routine MRI screening achieve detection of stage 0 or 1 tumours in over 90 per cent of cases, reducing the need for extensive, aggressive treatment and increasing the likelihood of breast-conserving therapy or less intensive interventions64–66.
Early diagnosis not only enhances survival, but also preserves quality of life by minimizing physical and psychological impacts associated with more advanced disease and harsher treatments.
Ezra’s MRI Scan with Spine costs £2,395 and is currently available at their partner clinic in Marylebone, London and in Sidcup, with more locations planned in the future. No referral is required, so you can book your scan directly without consulting a GP or specialist first. Most people pay out-of-pocket, as insurance typically does not cover self-referred scans, but you may be able to seek reimbursement depending on your policy.
Radiology reports generally have a standardised format to guide referring doctors and patients:
These questions will help you understand your results, clarify uncertainties, and participate in informed decision-making about your next steps.
Ezra provides a radiologist-reviewed report in a non-technical and easy-to-understand format on your dashboard.
MRI can detect cancer in many areas, especially soft tissues and organs, but it is not always the best test for every type of cancer and may miss smaller or certain types of tumours.
MRI does not use ionising radiation and has not been shown to cause cancer, making it one of the safest imaging techniques available.
An MRI can often distinguish between benign and malignant tumours based on certain imaging features, but a definite cancer diagnosis usually requires a biopsy or additional tests.
Cancerous tissue typically appears as a white or bright area compared to the surrounding tissue on standard black-and-white MRI scans, but contrast and colour can vary depending on scan settings or use of special dyes.
MRI results are not available immediately after the scan; images must be reviewed by a radiologist, and the final report is usually provided to your doctor within a few hours to a couple of days, unless it is an emergency.
MRI stands out for its safety, diagnostic performance in soft tissue tumours, and adaptability to emerging technologies, making it a cornerstone of modern and future cancer care.



1. Ghai S, Haider MA. Multiparametric-MRI in diagnosis of prostate cancer. Indian J Urol. 2015;31(3):194-201. doi:10.4103/0970-1591.159606
2. Gao Y, Reig B, Heacock L, et al. Magnetic Resonance Imaging in Screening of Breast Cancer. Radiol Clin North Am. 2021;59(1):85-98. doi:10.1016/j.rcl.2020.09.004
3. Sommer G, Wiese M, Winter L, et al. Preoperative staging of non-small-cell lung cancer: comparison of whole-body diffusion-weighted magnetic resonance imaging and 18F-fluorodeoxyglucose-positron emission tomography/computed tomography. Eur Radiol. 2012;22(12):2859-2867. doi:10.1007/s00330-012-2542-y
4. Popkin BM, D’Anci KE, Rosenberg IH. Water, Hydration and Health. Nutr Rev. 2010;68(8):439-458. doi:10.1111/j.1753-4887.2010.00304.x
5. Cancer Research UK. MRI scan. Accessed February 12, 2025. https://www.cancerresearchuk.org/about-cancer/tests-and-scans/mri-scan
6. DOE Explains...Protons. Energy.gov. Accessed March 14, 2025. https://www.energy.gov/science/doe-explainsprotons
7. Berger A. Magnetic resonance imaging. BMJ. 2002;324(7328):35.
8. Doria JJ. A Primer on Imaging. Alcohol Health Res World. 1995;19(4):261-265.
9. Amini B, Murphy WA, Haygood TM, et al. Gadolinium-based Contrast Agents Improve Detection of Recurrent Soft-Tissue Sarcoma at MRI. Radiol Imaging Cancer. 2020;2(2):e190046. doi:10.1148/rycan.2020190046
10. Grahl S, Bussas M, Pongratz V, et al. T1-Weighted Intensity Increase After a Single Administration of a Linear Gadolinium-Based Contrast Agent in Multiple Sclerosis. Clin Neuroradiol. 2021;31(1):235-243. doi:10.1007/s00062-020-00882-6
11. Raj H, Lal H, Rustagi S, et al. Biparametric magnetic resonance imaging as a diagnostic tool for differentiating RCC and renal pseudotumor in CKD patients. Urologia. 2025;92(2):201-208. doi:10.1177/03915603241276738
12. Ahlawat S, Fayad LM. Diffusion weighted imaging demystified: the technique and potential clinical applications for soft tissue imaging. Skeletal Radiol. 2018;47(3):313-328. doi:10.1007/s00256-017-2822-3
13. Ogris K, Petrovic A, Scheicher S, et al. Detection and volume estimation of artificial hematomas in the subcutaneous fatty tissue: comparison of different MR sequences at 3.0 T. Forensic Sci Med Pathol. 2017;13(2):135-144. doi:10.1007/s12024-017-9847-8
14. Santucci D, Faiella E, Cordelli E, et al. The Impact of Tumor Edema on T2-Weighted 3T-MRI Invasive Breast Cancer Histological Characterization: A Pilot Radiomics Study. Cancers (Basel). 2021;13(18):4635. doi:10.3390/cancers13184635
15. Mürtz P, Sprinkart AM, Block W, et al. Combined diffusion and perfusion index maps from simplified intravoxel incoherent motion imaging enable visual assessment of breast lesions. Sci Rep. 2025;15:17388. doi:10.1038/s41598-025-01984-2
16. Pinker K, Moy L, Sutton EJ, et al. Diffusion-weighted Imaging with Apparent Diffusion Coefficient Mapping for Breast Cancer Detection as a Stand-Alone-Parameter: Comparison with Dynamic Contrast-enhanced and Multiparametric Magnetic Resonance Imaging. Invest Radiol. 2018;53(10):587-595. doi:10.1097/RLI.0000000000000465
17. Sun SY, Ding Y, Li Z, et al. Multiparameter MRI Model With DCE-MRI, DWI, and Synthetic MRI Improves the Diagnostic Performance of BI-RADS 4 Lesions. Front Oncol. 2021;11:699127. doi:10.3389/fonc.2021.699127
18. Koh DM, Blackledge M, Padhani AR, et al. Whole-Body Diffusion-Weighted MRI: Tips, Tricks, and Pitfalls. American Journal of Roentgenology. 2012;199(2):252-262. doi:10.2214/AJR.11.7866
19. Chen H long, Zhou J qun, Chen Q, et al. Comparison of the sensitivity of mammography, ultrasound, magnetic resonance imaging and combinations of these imaging modalities for the detection of small (≤2 cm) breast cancer. Medicine (Baltimore). 2021;100(26):e26531. doi:10.1097/MD.0000000000026531
20. Virostko J, Hainline A, Kang H, et al. Dynamic contrast-enhanced magnetic resonance imaging and diffusion-weighted magnetic resonance imaging for predicting the response of locally advanced breast cancer to neoadjuvant therapy: a meta-analysis. J Med Imaging (Bellingham). 2018;5(1):011011. doi:10.1117/1.JMI.5.1.011011
21. Lord SJ, Lei W, Craft P, et al. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43(13):1905-1917. doi:10.1016/j.ejca.2007.06.007
22. Ghafoori M, Alavi M, Aliyari Ghasabeh M. MRI in Prostate Cancer. Iran Red Crescent Med J. 2013;15(12):e16620. doi:10.5812/ircmj.16620
23. Emir SN, Gürsu M, Bulut SSD. Evaluating the potential of abbreviated MRI protocols for liver metastasis detection: a study in colorectal cancer patients. Pol J Radiol. 2025;90:e19-e25. doi:10.5114/pjr/196906
24. Deike-Hofmann K, Thünemann D, Breckwoldt MO, et al. Sensitivity of different MRI sequences in the early detection of melanoma brain metastases. PLoS One. 2018;13(3):e0193946. doi:10.1371/journal.pone.0193946
25. Kim JH, Sun HY, Hwang J, et al. Diagnostic accuracy of contrast-enhanced computed tomography and contrast-enhanced magnetic resonance imaging of small renal masses in real practice: sensitivity and specificity according to subjective radiologic interpretation. World J Surg Oncol. 2016;14:260. doi:10.1186/s12957-016-1017-z
26. Petrén-Mallmin M, Andréasson I, Nyman R, et al. Detection of breast cancer metastases in the cervical spine. Acta Radiol. 1993;34(6):543-548.
27. Feng H, Shi G, Liu H, et al. The Application and Value of 3T Magnetic Resonance Imaging in the Display of Pulmonary Nodules. Front Oncol. 2022;12:844514. doi:10.3389/fonc.2022.844514
28. Macera A, Lario C, Petracchini M, et al. Staging of colorectal liver metastases after preoperative chemotherapy. Diffusion-weighted imaging in combination with Gd-EOB-DTPA MRI sequences increases sensitivity and diagnostic accuracy. Eur Radiol. 2013;23(3):739-747. doi:10.1007/s00330-012-2658-0
29. Dresen RC, De Vuysere S, De Keyzer F, et al. Whole-body diffusion-weighted MRI for operability assessment in patients with colorectal cancer and peritoneal metastases. Cancer Imaging. 2019;19:1. doi:10.1186/s40644-018-0187-z
30. Que FVF, Ishak NDB, Li ST, et al. Utility of Whole-Body Magnetic Resonance Imaging Surveillance in Children and Adults With Cancer Predisposition Syndromes: A Retrospective Study. JCO Precis Oncol. 2025;9:e2400642. doi:10.1200/PO-24-00642
31. Non-cancerous (benign) brain tumours. nhs.uk. March 4, 2025. Accessed July 22, 2025. https://www.nhs.uk/conditions/non-cancerous-benign-brain-tumours/
32. Tests to diagnose brain tumours. Accessed July 22, 2025. https://www.cancerresearchuk.org/about-cancer/brain-tumours/getting-diagnosed/what-are-the-tests
33. Lloyd C, McHugh K. The role of radiology in head and neck tumours in children. Cancer Imaging. 2010;10(1):49-61. doi:10.1102/1470-7330.2010.0003
34. Turek Ł, Sadowski M, Kurzawski J, et al. A 64-Year-Old Woman with Imaging Features Consistent with a Posterior Intrapericardial Lipoma and 5-Year Imaging Follow-Up. Am J Case Rep. 2021;22:e934500-1-e934500-5. doi:10.12659/AJCR.934500
35. Benign Soft Tissue Tumors. Cleveland Clinic. Accessed July 23, 2025. https://my.clevelandclinic.org/health/diseases/16778-benign-soft-tissue-tumors
36. Alamo L, Beck-Popovic M, Gudinchet F, et al. Congenital tumors: imaging when life just begins. Insights Imaging. 2011;2(3):297-308. doi:10.1007/s13244-011-0073-8
37. Chung WJ, Chung HW, Shin MJ, et al. MRI to differentiate benign from malignant soft-tissue tumours of the extremities: a simplified systematic imaging approach using depth, size and heterogeneity of signal intensity. Br J Radiol. 2012;85(1018):e831-e836. doi:10.1259/bjr/27487871
38. Bone Tumours. TeachMeSurgery. Accessed July 23, 2025. https://teachmesurgery.com/orthopaedic/principles/bone-tumours/
39. Canale S, Vilcot L, Ammari S, et al. Whole body MRI in paediatric oncology. Diagnostic and Interventional Imaging. 2014;95(6):541-550. doi:10.1016/j.diii.2013.11.002
40. Malherbe F, Nel D, Molabe H, et al. Palpable breast lumps: An age-based approach to evaluation and diagnosis. S Afr Fam Pract (2004). 2022;64(1):5571. doi:10.4102/safp.v64i1.5571
41. Salom M, Balacó I. How to distinguish a benign from a malignant tumour in children and when should a biopsy be done and by whom. EFORT Open Rev. 2024;9(5):393-402. doi:10.1530/EOR-24-0031
42. Henschke N, Maher CG, Ostelo RW, et al. Red flags to screen for malignancy in patients with low‐back pain. Cochrane Database Syst Rev. 2013;2013(2):CD008686. doi:10.1002/14651858.CD008686.pub2
43. Common Warning Signs of Brain Tumors. Accessed July 23, 2025. https://www.aaroncohen-gadol.com/en/patients/brain-tumor/natural-history/en/patients/brain-tumor/natural-history/warning-signs
44. Ahmed HU, El-Shater Bosaily A, Brown LC, et al. Diagnostic accuracy of multi-parametric MRI and TRUS biopsy in prostate cancer (PROMIS): a paired validating confirmatory study. Lancet. 2017;389(10071):815-822. doi:10.1016/S0140-6736(16)32401-1
45. Zhou B, He W, Kaur J, et al. Meta-Analysis of Abbreviated MRI Scanning Reveals a High Specificity and Sensitivity in Detecting Breast Cancer. Clinical and Experimental Obstetrics & Gynecology. 2023;50(6):115. doi:10.31083/j.ceog5006115
46. Teunissen WHT, Govaerts CW, Kramer MCA, et al. Diagnostic accuracy of MRI techniques for treatment response evaluation in patients with brain metastasis: A systematic review and meta-analysis. Radiother Oncol. 2022;177:121-133. doi:10.1016/j.radonc.2022.10.026
47. Gould MK, Donington J, Lynch WR, et al. Evaluation of Individuals With Pulmonary Nodules: When Is It Lung Cancer? Chest. 2013;143(5 Suppl):e93S-e120S. doi:10.1378/chest.12-2351
48. Sivathapandi T, Amalchandran J, Takalkar A, et al. Molecular Imaging of Lung and Pleural Tumors. In: Hall LT, ed. Molecular Imaging and Therapy. Exon Publications; 2023. Accessed July 23, 2025. http://www.ncbi.nlm.nih.gov/books/NBK599133/
49. Pandey A, Ghosh S, Prajapati P, et al. Pearls and Pitfalls in Applying PI-RADS 2. Journal of Gastrointestinal and Abdominal Radiology. 2024;07(03):183-202. doi:10.1055/s-0043-1778636
50. Krupa K, Bekiesińska-Figatowska M. Artifacts in Magnetic Resonance Imaging. Pol J Radiol. 2015;80:93-106. doi:10.12659/PJR.892628
51. Radzi S, Cowin G, Robinson M, et al. Metal artifacts from titanium and steel screws in CT, 1.5T and 3T MR images of the tibial Pilon: a quantitative assessment in 3D. Quantitative Imaging in Medicine and Surgery. 2014;4(3). doi:10.3978/j.issn.2223-4292.2014.03.06
52. Alghamdi SA. Gadolinium-Based Contrast Agents in Pregnant Women: A Literature Review of MRI Safety. Cureus. 15(5):e38493. doi:10.7759/cureus.38493
53. Radiology (ACR) RS of NA (RSNA) and AC of. Magnetic Resonance Imaging (MRI) - Head. Radiologyinfo.org. Accessed July 3, 2025. https://www.radiologyinfo.org/en/info/mri-brain
54. Williams JM, Hilmes MA, Archer B, et al. Repeatability and Reproducibility of Pancreas Volume Measurements Using MRI. Sci Rep. 2020;10:4767. doi:10.1038/s41598-020-61759-9
55. Gruber B, Froeling M, Leiner T, et al. RF coils: A practical guide for nonphysicists. J Magn Reson Imaging. 2018;48(3):590-604. doi:10.1002/jmri.26187
56. MRI scan. NHS inform. Accessed July 3, 2025. https://www.nhsinform.scot/tests-and-treatments/scans-and-x-rays/mri-scan/
57. Recommendations | Familial breast cancer: classification, care and managing breast cancer and related risks in people with a family history of breast cancer | Guidance | NICE. June 25, 2013. Accessed July 23, 2025. https://www.nice.org.uk/guidance/cg164/chapter/recommendations
58. Lowry KP, Lee JM, Kong CY, et al. Annual Screening Strategies in BRCA1 and BRCA2 Gene Mutation Carriers: A Comparative Effectiveness Analysis. Cancer. 2012;118(8):2021-2030. doi:10.1002/cncr.26424
59. Lim EJ, Leung C, Pitman A, et al. Magnetic resonance colonography for colorectal cancer screening in patients with Lynch syndrome gene mutation. Fam Cancer. 2010;9(4):555-561. doi:10.1007/s10689-010-9350-9
60. Kim DH, Choi SH, Shim JH, et al. Magnetic Resonance Imaging for Surveillance of Hepatocellular Carcinoma: A Systematic Review and Meta-Analysis. Diagnostics (Basel). 2021;11(9):1665. doi:10.3390/diagnostics11091665
61. Ali MU, Hussain SJ, Khalid M, et al. MRI-Driven Alzheimer’s Disease Diagnosis Using Deep Network Fusion and Optimal Selection of Feature. Bioengineering (Basel). 2024;11(11):1076. doi:10.3390/bioengineering11111076
62. Your child is having an MRI or CT scan under sedation. GOSH Hospital site. Accessed July 23, 2025. https://www.gosh.nhs.uk/conditions-and-treatments/procedures-and-treatments/your-child-having-mri-scan-under-sedation/
63. Alorainy IA, Albadr FB, Abujamea AH. Attitude towards MRI safety during pregnancy. Ann Saudi Med. 2006;26(4):306-309. doi:10.5144/0256-4947.2006.306
64. Baykara Ulusan M, Ferrara F, Meltem E, et al. MRI-only breast cancers are less aggressive than cancers identifiable on conventional imaging. European Journal of Radiology. 2024;181:111781. doi:10.1016/j.ejrad.2024.111781
65. Rahman WT, Gerard S, Grundlehner P, et al. Outcomes of High-Risk Breast MRI Screening in Women Without Prior History of Breast Cancer: Effectiveness Data from a Tertiary Care Center. J Breast Imaging. 2024;6(1):53-63. doi:10.1093/jbi/wbad092
66. Fields BKK, Joe BN. Screening Breast MRI Effectively Detects Early-Stage Breast Cancer in High-Risk Patients without Prior History of Breast Cancer. Radiol Imaging Cancer. 2024;6(2):e249005. doi:10.1148/rycan.249005
67. Nair A, Ong W, Lee A, et al. Enhancing Radiologist Productivity with Artificial Intelligence in Magnetic Resonance Imaging (MRI): A Narrative Review. Diagnostics. 2025;15(9):1146. doi:10.3390/diagnostics15091146
68. Din M, Daga K, Saoud J, et al. Clinicians’ perspectives on the use of artificial intelligence to triage MRI brain scans. European Journal of Radiology. 2025;183:111921. doi:10.1016/j.ejrad.2025.111921
69. Balchandani P, Naidich TP. Ultra-High-Field MR Neuroimaging. AJNR Am J Neuroradiol. 2015;36(7):1204-1215. doi:10.3174/ajnr.A4180
70. Trattnig S, Springer E, Bogner W, et al. Key clinical benefits of neuroimaging at 7 T. Neuroimage. 2018;168:477-489. doi:10.1016/j.neuroimage.2016.11.031
71. Kwee RM, Kwee TC. Whole‐body MRI for preventive health screening: A systematic review of the literature. J Magn Reson Imaging. 2019;50(5):1489-1503. doi:10.1002/jmri.26736