Magnetic resonance imaging (MRI) of the lungs is an advanced, radiation-free imaging technique that produces highly detailed images of the chest, with particular strength in visualising soft-tissue structures such as blood vessels, airways, the heart, and lymph nodes1. As technology evolves, lung MRI is emerging as a vital tool alongside low-dose CT, advancing both early diagnosis and strategies for personalised therapy in lung diseases.
This imaging technique is useful for tracking how cancer responds to treatment, allowing doctors to monitor whether a tumour is shrinking or not. MRI’s excellent soft-tissue contrast often complements CT findings and guides therapy decisions2.
Newer techniques, such as diffusion-weighted imaging (DWI) and perfusion mapping, are making lung MRIs even more powerful. Meanwhile, AI-radiomics is helping to push MRI to the forefront for early detection and supporting truly personalised therapy3.
Learn more about MRI for cancer detection here.
There are several reasons why a lung MRI might be ordered, including:
If a previous CT scan has identified a small (≤8 mm) ground-glass or sub-solid nodule, especially one labelled as Lung-RADS 3-4A, a lung MRI can provide detailed insight into its diffusion, perfusion, and margins4,5. This helps guide biopsy decisions by better distinguishing benign from potentially malignant lesions.
For patients with a known lung tumour, MRI excels at clarifying whether cancer has invaded the chest wall, mediastinum, or major vessels, such as the aorta, pulmonary artery, or superior vena cava5,6. High-resolution soft-tissue imaging is crucial for surgical planning, enabling surgeons to determine whether less invasive surgery is feasible or if open surgery should be avoided.
Lung MRI is especially valuable in patients at higher long-term cancer risk, such as heavy smokers in screening programs, those with a history of chest irradiation, people with genetic cancer syndromes (like Li-Fraumeni or EGFR mutations), or individuals with asbestos exposure1,7. In these groups, MRI’s absence of radiation provides a safer option for repeated surveillance or follow-up imaging.
For staging lung cancer and detecting distant spread, whole-body MRI can identify small pleural, vertebral, or brain metastases that may escape detection on CT or PET/CT5,6. Identifying additional metastatic sites can significantly impact the treatment plan, informing whether curative or systemic therapy is most suitable.
When symptoms such as persistent hemoptysis (coughing up blood), unexplained chest pain, or chronic cough remain unresolved despite X-ray or low-dose CT, MRI provides additional clarification7,8. It is particularly helpful when distinguishing between inflammatory disorders and tumours, leading to more informed diagnoses.
MRI can track changes in tumour size (such as RECIST criteria), as well as more advanced markers like apparent diffusion coefficient (ADC) and perfusion after treatment with chemotherapy, immunotherapy, stereotactic radiotherapy (SBRT), ablation, or targeted drugs9. This enables more precise and early assessment of how a cancer is responding to therapy.
MRI is the imaging modality of choice for patients who are pregnant, paediatric, frail, or otherwise unable to tolerate ionising radiation1,7,10. Special respiratory-gated or ultra-short echo time (UTE) MRI sequences can produce diagnostic-quality images even in these especially sensitive situations, reducing the risks associated with traditional scans.
You can use our Know Your Risk Calculator to understand your risk of cancer in just five minutes.
Here are a few tips to help you prepare for your MRI11:
You can read more about preparation for Ezra’s Full Body Scan here.
Upon arrival for your MRI, you will need to check in and complete a screening form. This will allow you to confirm the presence of implants, allergies, and whether you might need any anxiety medication.
During the scan, you will lie down on a sliding table. A dedicated surface or phased-array coil is typically placed over the limb or region of interest13. The scan typically lasts 30-45 minutes of actual “table time”, during which the technician may acquire multiple sequences (settings). If contrast is used, you might feel a cool sensation in the arm.
You’ll hear a series of loud knocking or tapping sounds as the MRI machine works. This is completely normal. The scan usually takes about 20 to 45 minutes, and you’ll be offered earplugs or headphones to make the experience more comfortable.
You’ll stay in touch with the team via a two-way intercom and a squeeze bulb, allowing you to communicate or pause the scan if needed. If contrast is required, it’s injected halfway through, possibly causing a brief cool sensation. After the final sequence, the coil is removed, and you’re free to go.
At Ezra, our Full Body Plus scan takes around 60 minutes total, with 45 minutes of table time. Earplugs or headphones are available.
MRI is generally considered very safe when proper screening and protocols are followed, but certain risks and side effects should be understood:
A deeper dive into possible side effects (such as heat, headaches, and gadolinium deposition) is available in our full guide.
At Ezra, we employ a contrast-free approach using wide-bore T3 machines to deliver a comfortable scanning experience.
MRI reports for lung scans often include specialised terms. Here’s a guide to some common phrases and what they mean for your diagnosis:
Ezra provides a radiologist-reviewed report in a non-technical and easy-to-understand format on your dashboard.
After the MRI scan, you will be free to go home and continue with your day without any precautions27. If you received a sedative, you will need another person to pick you up. You will also not be able to drive, consume alcohol or operate heavy machinery 24 hours after the sedative.
A team of experts will review your results and determine whether a follow-up is necessary and recommend the appropriate treatment if needed. If abnormalities are found, you may undergo ongoing monitoring every 2-3 months to track recurrence. You can receive support in the form of counselling and advice on how to handle aspects like claustrophobia.
If you have a scan with us here at Ezra, you will receive your report within five to seven days and have the option to discuss it with a medical practitioner. You can also access your scan images through the online portal.
MRI provides precise measurements of lung tumour size and shape, distinguishing between smooth margins (often benign or indolent) and spiculated, jagged margins, which are closely linked to a higher risk of malignancy and a worse prognosis28,29. Spiculated or irregular margins also carry independent prognostic information, aiding both accurate staging and risk prediction.
A key strength of MRI lies in its ability to reveal how the tumour relates to nearby structures such as major blood vessels, airways, and the pleura6,30. Clear imaging of tumour extension or invasion determines whether the lesion can be safely removed by surgery or if vital structures are involved, making surgery unfeasible.
MRI can detect central areas of necrosis (dead tissue) or cavitation (air-filled spaces within a tumour), findings that are particularly common in squamous cell lung cancers31,32. The presence of cavitation may change biopsy plans and influence treatment by indicating aggressive biology or past therapy effects, especially in patients receiving anti-angiogenic drugs.
Diffusion-weighted MRI allows for quantification of how easily water molecules move within tumour tissue, summarised as the ADC. Low ADC values (typically less than 1.0 x 10⁻³ mm²/s) signal high cellular density, an indicator of aggressive, poorly differentiated cancers, and are associated with worse histologic grade and higher tumour aggressiveness33,34.
With dynamic contrast-enhanced MRI, tumours can be evaluated for how quickly and how much they “light up” after contrast injection. Malignant tumours often demonstrate rapid early enhancement with a plateau or quick washout, while a slower, progressive pattern is more characteristic of scar tissue or inflammation, helping to differentiate malignant from benign lesions35,36.
Advanced MRI techniques can assess the variability in blood flow (perfusion heterogeneity) throughout the tumour. Regions with poor perfusion often correspond to hypoxic areas, subsets of tumours that may be more resistant to chemotherapy and radiotherapy, providing important information for individualising treatment37,38.
Ezra utilises DWI as part of our whole-body MRI scans and artificial intelligence (AI) to enhance MRI images and convert radiology reports into layman's term translations.
Adenocarcinoma (Solid/Glandular): Lung adenocarcinomas can present as small pulmonary nodules with high tissue-contrast resolution on MRI. Tumour components exhibit variable signal and enhancement patterns: aggregated tumour cells often display marked early enhancement, while fibrotic components are typically hypointense, and necrotic areas are hyperintense on T2-weighted images39.
Squamous-Cell Carcinoma: Squamous-cell carcinoma tends to occur centrally in the lungs and often manifests as a mass of central cavitation40. On MRI and perfusion studies, it usually shows strong enhancement and intense signal on DWI.
Small-Cell Lung Cancer: Small-cell lung cancer (SCLC) typically presents as a bulky hilar or mediastinal mass. On MRI, SCLC shows very low ADC values, which are often associated with extensive nodal spread at the time of diagnosis41.
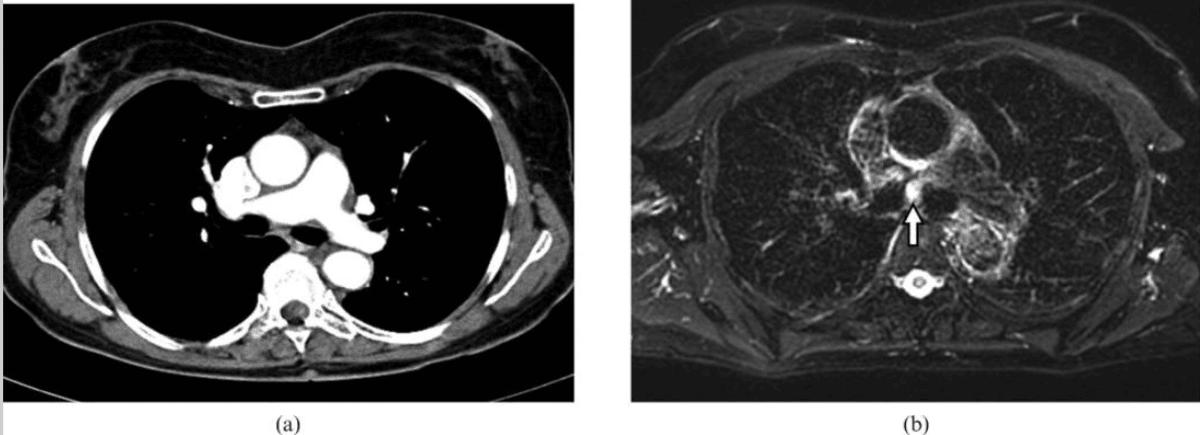
Carcinoid Tumour: These tumours are generally well-defined, lobulated nodules that show strong, even contrast enhancement on MRI42. These tumours are highly vascular but tend not to invade adjacent structures, and their smooth borders help differentiate them from more aggressive cancers.
Mucinous Tumours: Mucinous lung tumours (invasive mucinous adenocarcinomas) characteristically display high signal on T2-weighted MRI due to their mucin-rich content43,44. They may also exhibit raised proton-density fat-fraction (PDFF) on specialised MRI sequences, and frequently show ground-glass or consolidative opacities with air bronchogram.
Ezra screens for over 500 conditions, including lung abnormalities.
There are multiple types of MRI scans, all using different methods to give a better visualisation of lung tumours.
Ezra uses whole-body DWI imaging to get a full picture of the body and catch any potential abnormalities.
Ezra’s MRI Scan with Spine costs £2,395 and is currently available at their partner clinic in Marylebone, London and in Sidcup, with more locations planned in the future. You can also add a lung CT for £249 to look for lung cancer, pulmonary nodules, emphysema, and more. No referral is required, so you can book your scan directly without consulting a GP or specialist first. Most people pay out of pocket, as insurance typically does not cover self-referred scans, but you may be able to seek reimbursement depending on your policy.
Typically, only the chest needs to be imaged for a lung MRI; however, if a whole-body disease assessment or metastasis survey is required, your entire body may be scanned.
Yes, MRI can detect lung cancer and is especially useful for characterising tumours, assessing their relationships to surrounding structures, and staging, though low-dose CT remains the gold standard for initial detection.
CT is generally superior to MRI for detecting lung cancer and nodules due to its higher sensitivity and specificity; however, MRI offers advantages for evaluating soft tissue and in certain clinical scenarios.
MRI can reliably detect lung nodules larger than 4–6 mm and performs almost as well as CT for nodules 8–10 mm or larger; however, it is less sensitive for smaller nodules.
Ezra uses both MRI and CT because MRI provides comprehensive, radiation-free imaging of multiple organs, while low-dose CT remains best for lung cancer screening and evaluating certain conditions that MRI cannot assess as well.



1. Biederer J, Mirsadraee S, Beer M, et al. MRI of the lung (3/3)—current applications and future perspectives. Insights Imaging. 2012;3(4):373-386. doi:10.1007/s13244-011-0142-z
2. Usuda K, Iwai S, Funasaki A, et al. Diffusion-weighted magnetic resonance imaging is useful for the response evaluation of chemotherapy and/or radiotherapy to recurrent lesions of lung cancer. Transl Oncol. 2019;12(5):699-704. doi:10.1016/j.tranon.2019.02.005
3. Binczyk F, Prazuch W, Bozek P, et al. Radiomics and artificial intelligence in lung cancer screening. Transl Lung Cancer Res. 2021;10(2):1186-1199. doi:10.21037/tlcr-20-708
4. Biederer J, Hintze C, Fabel M. MRI of pulmonary nodules: technique and diagnostic value. Cancer Imaging. 2008;8(1):125-130. doi:10.1102/1470-7330.2008.0018
5. Hochhegger B, Marchiori E, Sedlaczek O, et al. MRI in lung cancer: a pictorial essay. Br J Radiol. 2011;84(1003):661-668. doi:10.1259/bjr/24661484
6. Khalil A, Bouhela T, Carette M. Contribution of MRI in lung cancer staging. Journal of the Belgian Society of Radiology. 2013;96(3):132. doi:10.5334/jbr-btr.234
7. MRI of the lungs and bronchi - what the study shows, its indications and benefits. August 24, 2023. Accessed July 17, 2025. https://medconsonline.com/en/blog/lung-and-bronchi-mri
8. Kauczor HU, Ley-Zaporozhan J, Ley S. Imaging of Pulmonary Pathologies. Proc Am Thorac Soc. 2009;6(5):458-463. doi:10.1513/pats.200901-002AW
9. Bainbridge H, Salem A, Tijssen RHN, et al. Magnetic resonance imaging in precision radiation therapy for lung cancer. Transl Lung Cancer Res. 2017;6(6):689-707. doi:10.21037/tlcr.2017.09.02
10. Cobben DCP, de Boer HCJ, Tijssen RH, et al. Emerging Role of MRI for Radiation Treatment Planning in Lung Cancer. Technol Cancer Res Treat. 2016;15(6):NP47-NP60. doi:10.1177/1533034615615249
11. Radiology (ACR) RS of NA (RSNA) and AC of. Magnetic Resonance Imaging (MRI) - Head. Radiologyinfo.org. Accessed July 3, 2025. https://www.radiologyinfo.org/en/info/mri-brain
12. Williams JM, Hilmes MA, Archer B, et al. Repeatability and Reproducibility of Pancreas Volume Measurements Using MRI. Sci Rep. 2020;10:4767. doi:10.1038/s41598-020-61759-9
13. Gruber B, Froeling M, Leiner T, et al. RF coils: A practical guide for nonphysicists. J Magn Reson Imaging. 2018;48(3):590-604. doi:10.1002/jmri.26187
14. Gill A, Shellock FG. Assessment of MRI issues at 3-Tesla for metallic surgical implants: findings applied to 61 additional skin closure staples and vessel ligation clips. J Cardiovasc Magn Reson. 2012;14(1):3. doi:10.1186/1532-429X-14-3
15. Potential Hazards and Risks. UCSF Radiology. January 20, 2016. Accessed March 14, 2025. https://radiology.ucsf.edu/patient-care/patient-safety/mri/potential-hazards-risks
16. Costello JR, Kalb B, Martin DR. Incidence and Risk Factors for Gadolinium-Based Contrast Agent Immediate Reactions. Top Magn Reson Imaging. 2016;25(6):257-263. doi:10.1097/RMR.0000000000000109
17. McDonald RJ, McDonald JS, Kallmes DF, et al. Gadolinium Deposition in Human Brain Tissues after Contrast-enhanced MR Imaging in Adult Patients without Intracranial Abnormalities. Radiology. 2017;285(2):546-554. doi:10.1148/radiol.2017161595
18. O’Dowd EL, Tietzova I, Bartlett E, et al. ERS/ESTS/ESTRO/ESR/ESTI/EFOMP statement on management of incidental findings from low dose CT screening for lung cancer. European Journal of Cardio-Thoracic Surgery. 2023;64(4):ezad302. doi:10.1093/ejcts/ezad302
19. Mall MA, Stahl M, Graeber SY, et al. Early detection and sensitive monitoring of CF lung disease: Prospects of improved and safer imaging. Pediatr Pulmonol. 2016;51(S44):S49-S60. doi:10.1002/ppul.23537
20. Bankier AA, MacMahon H, Colby T, et al. Fleischner Society: Glossary of Terms for Thoracic Imaging. Radiology. 2024;310(2):e232558. doi:10.1148/radiol.232558
21. Aslan A, İnan İ, Aktan A, et al. The utility of ADC measurement techniques for differentiation of low- and high-grade clear cell RCC. Pol J Radiol. 2018;83:e446-e451. doi:10.5114/pjr.2018.80207
22. Wang D, Liu S, Fu J, et al. Correlation of Ktrans derived from dynamic contrast-enhanced MRI with treatment response and survival in locally advanced NSCLC patients undergoing induction immunochemotherapy and concurrent chemoradiotherapy. J Immunother Cancer. 2024;12(6):e008574. doi:10.1136/jitc-2023-008574
23. Chen L, Zeng X, Wu Y, et al. A Study of the Correlation of Perfusion Parameters in High-Resolution GRASP MRI With Microvascular Density in Lung Cancer. J Magn Reson Imaging. 2019;49(4):1186-1194. doi:10.1002/jmri.26340
24. Morgan MA. Lung Imaging Reporting and Data System (Lung-RADS) | Radiology Reference Article | Radiopaedia.org. Radiopaedia. doi:10.53347/rID-32681
25. Jiang B, Han D, van der Aalst CM, et al. Lung cancer volume doubling time by computed tomography: A systematic review and meta-analysis. European Journal of Cancer. 2024;212:114339. doi:10.1016/j.ejca.2024.114339
26. Kalra MK, Karout L, Kiipper F de M, et al. Decoding dose descriptors for computed tomography. Radiol Bras. 57:e20230116. doi:10.1590/0100-3984.2023.0116
27. MRI scan. NHS inform. Accessed July 3, 2025. https://www.nhsinform.scot/tests-and-treatments/scans-and-x-rays/mri-scan/
28. Lee G, Park H, Lee HY, et al. Tumor Margin Contains Prognostic Information: Radiomic Margin Characteristics Analysis in Lung Adenocarcinoma Patients. Cancers (Basel). 2021;13(7):1676. doi:10.3390/cancers13071676
29. Yi CA, Jeon TY, Lee KS, et al. 3-T MRI: Usefulness for Evaluating Primary Lung Cancer and Small Nodules in Lobes Not Containing Primary Tumors. American Journal of Roentgenology. 2007;189(2):386-392. doi:10.2214/AJR.07.2082
30. Purandare NC, Rangarajan V. Imaging of lung cancer: Implications on staging and management. Indian J Radiol Imaging. 2015;25(2):109-120. doi:10.4103/0971-3026.155831
31. Calvetti L, Aprile G. Influence and mechanism of lung cavitation development on antiangiogenic therapy: is cavitation the new caveat? Transl Lung Cancer Res. 2019;8(4):319-322. doi:10.21037/tlcr.2019.08.19
32. Nishino M, Cryer SK, Okajima Y, et al. Tumoral cavitation in patients with non-small-cell lung cancer treated with antiangiogenic therapy using bevacizumab. Cancer Imaging. 2012;12(1):225-235. doi:10.1102/1470-7330.2012.0027
33. Liu H, Liu Y, Yu T, et al. Evaluation of apparent diffusion coefficient associated with pathological grade of lung carcinoma, before therapy. Journal of Magnetic Resonance Imaging. 2015;42(3):595-601. doi:10.1002/jmri.24823
34. Zhang F, Zhou Z, Tang D, et al. Diffusion-weighted MRI in solitary pulmonary lesions: associations between apparent diffusion coefficient and multiple histopathological parameters. Sci Rep. 2018;8(1):11248. doi:10.1038/s41598-018-29534-z
35. Donmez FY, Yekeler E, Saeidi V, et al. Dynamic Contrast Enhancement Patterns of Solitary Pulmonary Nodules on 3D Gradient-Recalled Echo MRI. American Journal of Roentgenology. 2007;189(6):1380-1386. doi:10.2214/AJR.07.2429
36. Yoo MR, Whang SH, Park CH, et al. Dynamic Contrast-Enhanced CT in Advanced Lung Cancer after Chemotherapy with/without Radiation Therapy: Can It Predict Treatment Responsiveness of the Tumor? Journal of the Korean Society of Radiology. 2013;69(2):131-138. doi:10.3348/jksr.2013.69.2.131
37. Tao X, Wang L, Hui Z, et al. DCE-MRI Perfusion and Permeability Parameters as predictors of tumor response to CCRT in Patients with locally advanced NSCLC. Sci Rep. 2016;6(1):35569. doi:10.1038/srep35569
38. Bashir U, Siddique MM, Mclean E, et al. Imaging Heterogeneity in Lung Cancer: Techniques, Applications, and Challenges. American Journal of Roentgenology. 2016;207(3):534-543. doi:10.2214/AJR.15.15864
39. Awaya H, Matsumoto T, Honjo K, et al. A preliminary study of discrimination among the components of small pulmonary nodules by MR imaging: correlation between MR images and histologic appearance. Radiat Med. 2000;18(1):29-38.
40. Yang M, Shi L, Huang T, et al. Value of contrast-enhanced magnetic resonance imaging-T2WI-based radiomic features in distinguishing lung adenocarcinoma from lung squamous cell carcinoma with solid components >8 mm. Journal of Thoracic Disease. 2023;15(2). doi:10.21037/jtd-23-142
41. Christensen TN, Langer SW, Villumsen KE, et al. 18F-fluorothymidine (FLT)-PET and diffusion-weighted MRI for early response evaluation in patients with small cell lung cancer: a pilot study. Eur J Hybrid Imaging. 2020;4:2. doi:10.1186/s41824-019-0071-5
42. Megahed M, Sharif R, Abdeen Y. Synchronous bilateral typical pulmonary carcinoid tumours diagnosed by robotic navigation bronchoscopy: A unique case. Respirol Case Rep. 2024;12(11):e70055. doi:10.1002/rcr2.70055
43. Pan X, Fang R, Zhang B, et al. Pathological and imaging features of pulmonary invasive mucinous adenocarcinoma—a retrospective cohort study. Transl Lung Cancer Res. 2024;13(6):1376-1382. doi:10.21037/tlcr-24-526
44. Ogusu S, Takahashi K, Hirakawa H, et al. Primary Pulmonary Colloid Adenocarcinoma: How Can We Obtain a Precise Diagnosis? Intern Med. 2018;57(24):3637-3641. doi:10.2169/internalmedicine.1153-18
45. Wang YXJ, Lo GG, Yuan J, et al. Magnetic resonance imaging for lung cancer screen. J Thorac Dis. 2014;6(9):1340-1348. doi:10.3978/j.issn.2072-1439.2014.08.43
46. Broncano J, Steinbrecher K, Marquis KM, et al. Diffusion-weighted Imaging of the Chest: A Primer for Radiologists. RadioGraphics. 2023;43(7):e220138. doi:10.1148/rg.220138
47. Hatzoglou V, Tisnado J, Mehta A, et al. Dynamic contrast‐enhanced MRI perfusion for differentiating between melanoma and lung cancer brain metastases. Cancer Med. 2017;6(4):761-767. doi:10.1002/cam4.1046
48. Guo W, Lv B, Yang T, et al. Role of Dynamic Contrast-Enhanced Magnetic Resonance Imaging Parameters and Extracellular Volume Fraction as Predictors of Lung Cancer Subtypes and Lymph Node Status in Non-Small-Cell Lung Cancer Patients. J Cancer. 2023;14(16):3108-3116. doi:10.7150/jca.88367
49. Takahashi M, Togao O, Obara M, et al. Ultra-short echo time (UTE) MR imaging of the lung: Comparison between normal and emphysematous lungs in mutant mice. J Magn Reson Imaging. 2010;32(2):326-333. doi:10.1002/jmri.22267
50. Renz DM, Herrmann KH, Kraemer M, et al. Ultrashort echo time MRI of the lung in children and adolescents: comparison with non-enhanced computed tomography and standard post-contrast T1w MRI sequences. Eur Radiol. 2022;32(3):1833-1842. doi:10.1007/s00330-021-08236-7
51. nihr_wp. Whole-body MRI scans are as accurate as standard imaging pathways for lung cancer staging. NIHR Evidence. doi:10.3310/signal-000796